Testing of chemicals
Adverse Outcome Pathways, Molecular Screening and Toxicogenomics
The OECD Environmental, Health and Safety (EHS) Programme has been helping member countries to make better use of increased knowledge of how chemicals induce adverse effects in humans and wildlife, through the so-called Adverse Outcome Pathways.
What's new?
|
|
In October 2022, two scientific journals, respectively ALTEX - Alternatives to Animal Experimentation and Cancers signed a Memorandum of Understanding (MOU) with the OECD on co-operative activities in the field of Adverse Outcome Pathway (AOP) development, in order to increase the number of AOPs developed, reviewed and published. In total, the OECD has now signed a MOU with four scientific journals and is seeking more journals to join in. More information is available here. |
What is an Adverse Outcome Pathway?
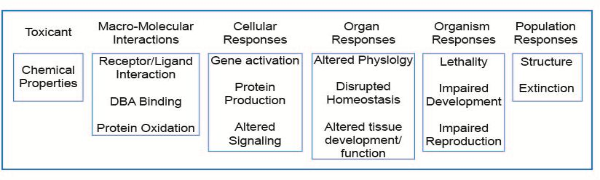
The OECD launched a new programme on the development of Adverse Outcome Pathways (AOP) in 2012. An AOP is an analytical construct that describes a sequential chain of causally linked events at different levels of biological organisation that lead to an adverse health or ecotoxicological effect (see figure below). AOPs are the central element of a toxicological knowledge framework being built to support chemical risk assessment based on mechanistic reasoning.
Schematic representation of the AOP illustrated with reference to a number of pathways
Watch our video: OECD work on Adverse Outcome Pathway
Publications on Adverse Outcome Pathways
Access all publications on Adverse Outcome Pathways. Find the OECD Series on Adverse Outcome Pathways on iLibrary  .
.
The AOP Development Programme
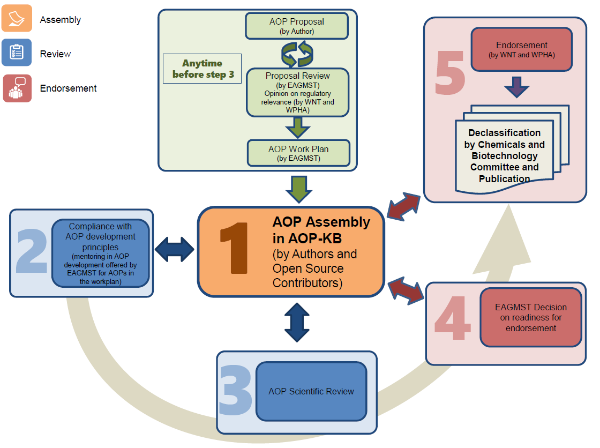
The AOP development programme is overseen by the Extended Advisory Group on Molecular Screening and Toxicogenomics (EAGMST). EAGMST members play an active role in the development of AOPs and can provide support to AOP authors during the development of an AOP in the AOP Wiki.
- General Guidance on AOP is provided in the Guidance Document on Developing and Assessing Adverse Outcome Pathways
- A new online version of the Developers' Handbook is available from the AOP-Wiki. It provides practical guidance and should be followed for developing an AOP in the AOP-Wiki platform.
- AOP-relevant projects can be proposed by scientists from OECD member countries for inclusion in the OECD AOP Development Programme work plan. Complete the form to submit an AOP project proposal.
- The Guidance Document for the scientific review of Adverse Outcome Pathways provides OECD standards for the scientific review of an AOP in the AOP Wiki.
- The AOP work plan is updated in July and January every year.
- The AOP-Wiki also provides AOP-related training resources, i.e. online training and training events.
Representation of the AOP Development Process at the OECD
Cooperation on scientific review and AOP publication
The OECD is developing co-operation with Scientific Journals for the review and publication of AOPs.
This cooperation is formalised in the signature of a Memorandum of understanding (MOU). Scientific Journal Signatories take part in the scientific review of AOPs relevant to their field of expertise, with the view to achieve the following common objectives:
- Increase the rate of AOP scientific reviews;
- Offer the opportunity for journals to get more submissions for scientific articles;
- Enable a double recognition through AOP publications in the scientific literature and at the OECD.
The conditions of the MOU are summarised here.
The following Journals have already signed an MOU with the OECD:
- Environmental Toxicology and Chemistry (ET&C), a journal published by the Society of Environmental Toxicology and Chemistry (SETAC)
- AOP Pathways and Predictions: Virtual issue - Environmental and Molecular Mutagenesis (EMM), the journal of the Environmental Mutagenesis and Genomics Society
- Alternatives to Animal Experimentation (ALTEX)
- Cancers, a journal published by MDPI
The OECD's AOP Knowledge Base tools, constantly developed and refined, are a web-based platform which aims to bring together all knowledge on how chemicals can induce adverse effects, therefore providing a focal point for AOP development and dissemination.
|
|
|
|
Missed our previous webinars on AOPs? Watch the video recordings
- Adverse Outcome Pathway co-operative activities between Scientific journals and the OECD - 25 January 2022
The objective of this webinar, dedicated to journal editors or publishers interested in reviewing/publishing AOPs, was to present the basis for cooperation between scientific journals and the OECD and discuss the lessons learnt so far.
- Training needs, resources and opportunities for adverse outcome pathways (AOPs) - 30 November 2020
This interactive webinar discussed opportunities for expanding the AOP community of trainers to meet current needs, considering all available resources. - Adverse Outcome Knowledge Base (AOP-KB) and AOP developing tips - Thursday 30 January 2020
The third webinar on Adverse Outcome Pathways (AOPs) focused on the core information and telecommunication technologies applications that were built to support AOP development, management and dissemination. Tools and technical tips to facilitate scientific knowledge assembling and evaluations were also presented. The AOP framework implements a collaborative and innovative approach for collecting mechanistic knowledge from various sources that can eventually support chemical safety assessment. - Adverse Outcome Pathways: Assembling and evaluating weight of evidence and quantitative understanding - Wednesday 15 January 2020
This second webinar on Adverse Outcome Pathways (AOPs) focused on the importance of weight of evidence in the process of developing AOPs, the types and lines of evidences assembled, examples demonstrating the lines of evidence and understanding why quantitative AOPs are developed. The AOP framework is a collaborative tool that applies an innovative approach for collecting mechanistic knowledge from various sources that can eventually support chemical safety assessment. - Adverse Outcome Pathways Framework - Tuesday 30 April 2019
This first webinar on Adverse Outcome Pathways (AOPs) discussed the Adverse Outcome Pathway (AOP) framework. The AOP framework is a collaborative tool that applies an innovative approach for collecting mechanistic knowledge from various sources that can eventually support chemical safety assessment. - Access all the presentations
Find out more
| Test Guidelines Programme The OECD Test Guidelines Programme for the identification of new biomaker endpoints and in vitro test methods that are candidates to become part of OECD Test Guidelines. |
Hazard Assessment Activities |
ENGAGE WITH US
- To receive our latest news, publications and events, sign up to the Chemical Safety and Biotechnology Update newsletters.
- Contact: [email protected]
- Stay tuned on Twitter: @OECD_ENV
- Watch our videos on our YouTube channel
Related Documents

